Self-assembled palladium nanoflowers supported on fullerene: Electrochemical catalytic performance for the reduction of 4-nitrophenol - ScienceDirect

On the Mechanism of Palladium-Catalyzed Aromatic C−H Oxidation | Journal of the American Chemical Society
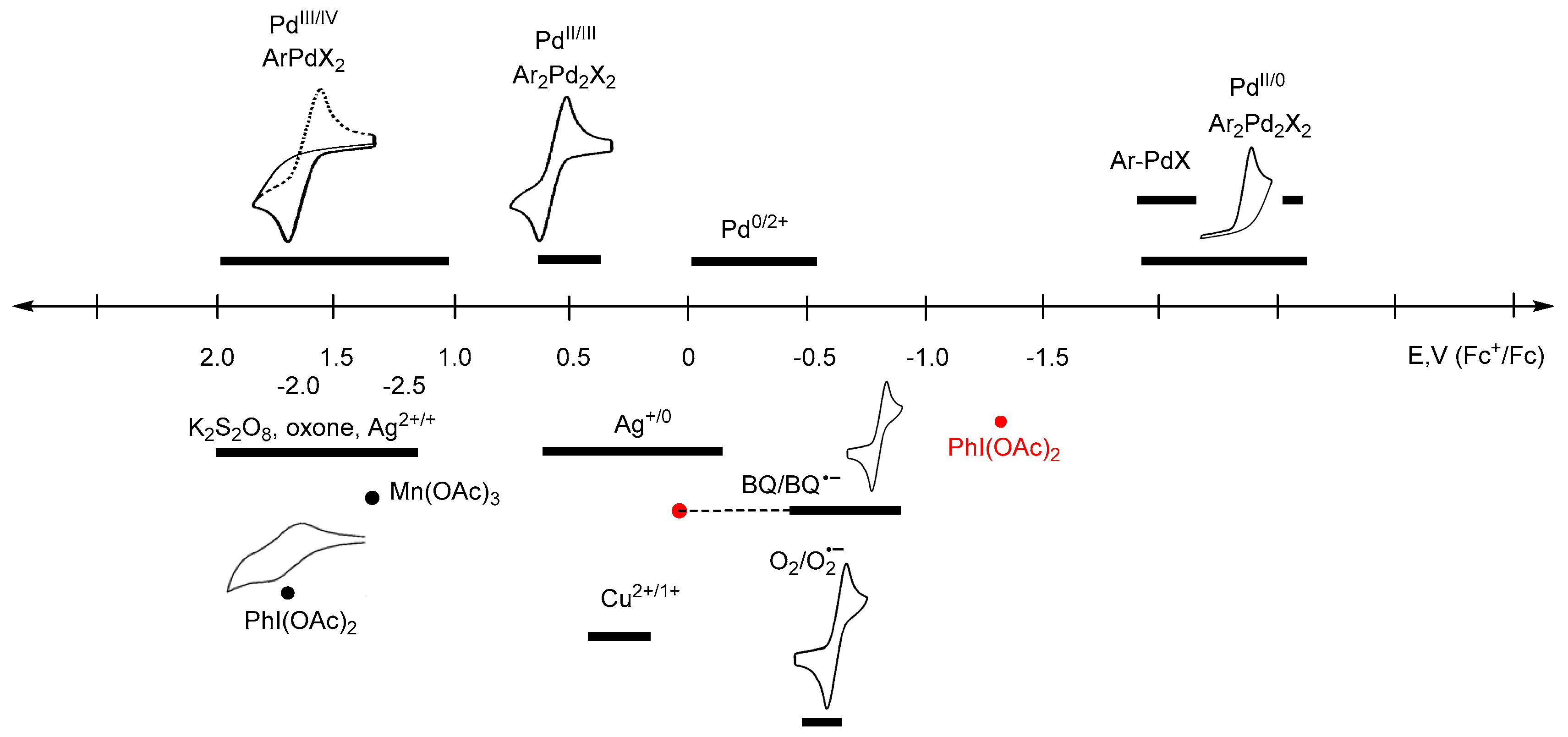
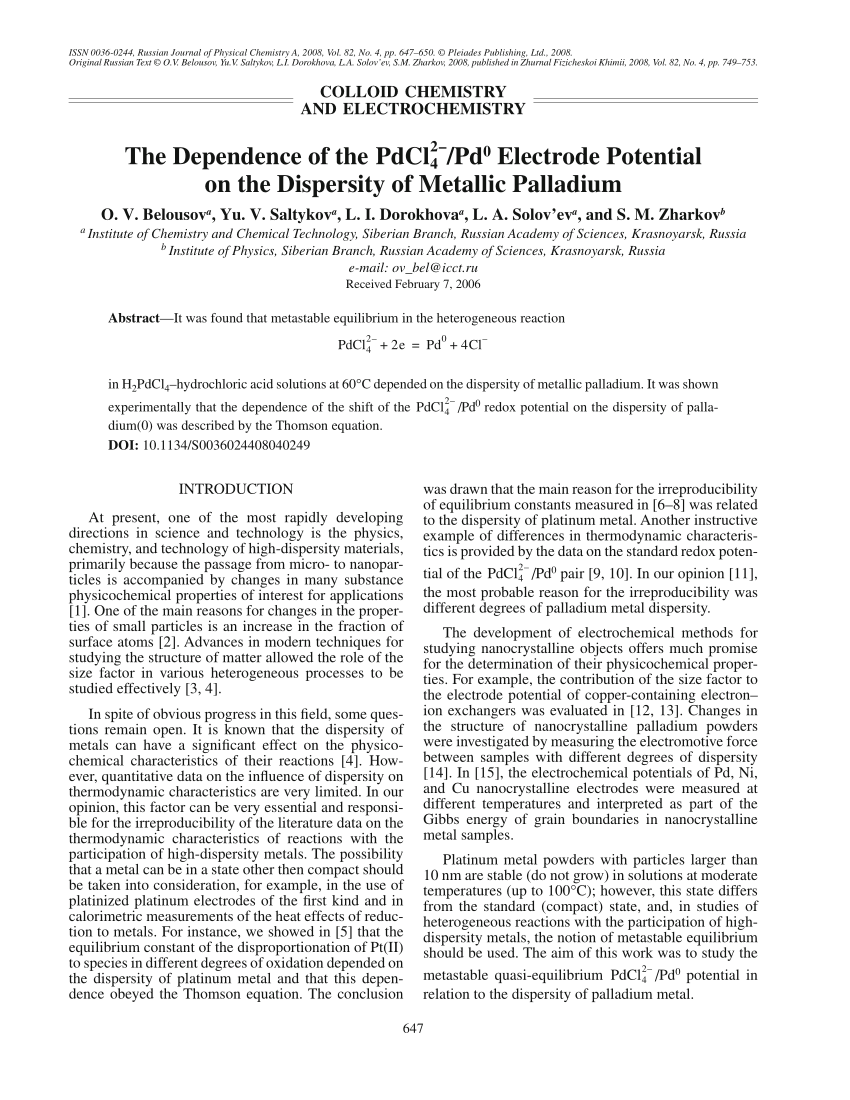
Inorganics | Free Full-Text | Redox-Induced Aromatic C–H Bond Functionalization in Metal Complex Catalysis from the Electrochemical Point of View

Electrochemical behavior of CO2 reduction on palladium nanoparticles: Dependence of adsorbed CO on electrode potential - ScienceDirect

Can Donor Ligands Make Pd(OAc)2 a Stronger Oxidant? Access to Elusive Palladium(II) Reduction Potentials and Effects of Ancillary Ligands via Palladium(II)/Hydroquinone Redox Equilibria | Journal of the American Chemical Society

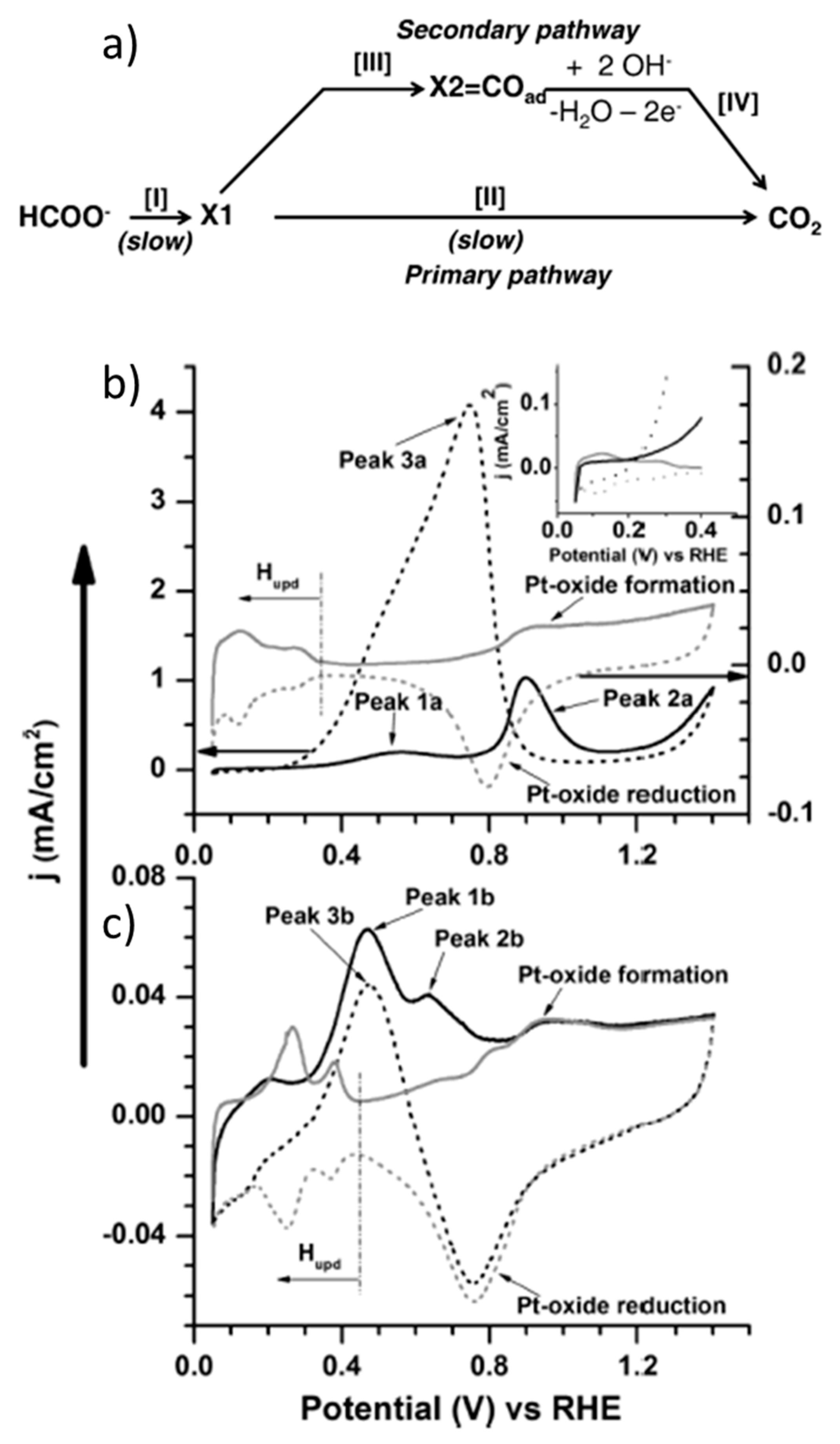
Partial Palladium Oxidation over Various Oxide Supports for a Higher Reactivity of PdO with Respect to CH4 | The Journal of Physical Chemistry C

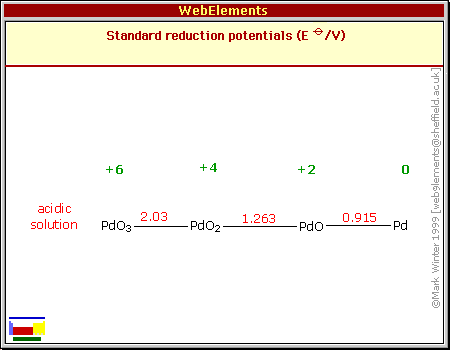
Redox trends in cyclometalated palladium( ii ) complexes - Dalton Transactions (RSC Publishing) DOI:10.1039/C6DT03786K
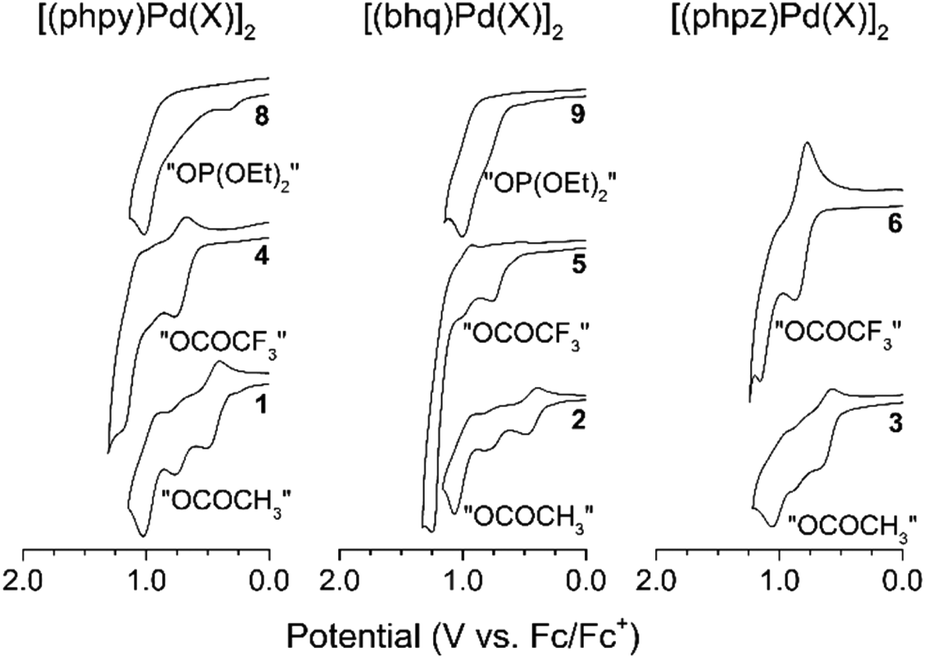
Redox trends in cyclometalated palladium( ii ) complexes - Dalton Transactions (RSC Publishing) DOI:10.1039/C6DT03786K

Redox trends in cyclometalated palladium( ii ) complexes - Dalton Transactions (RSC Publishing) DOI:10.1039/C6DT03786K
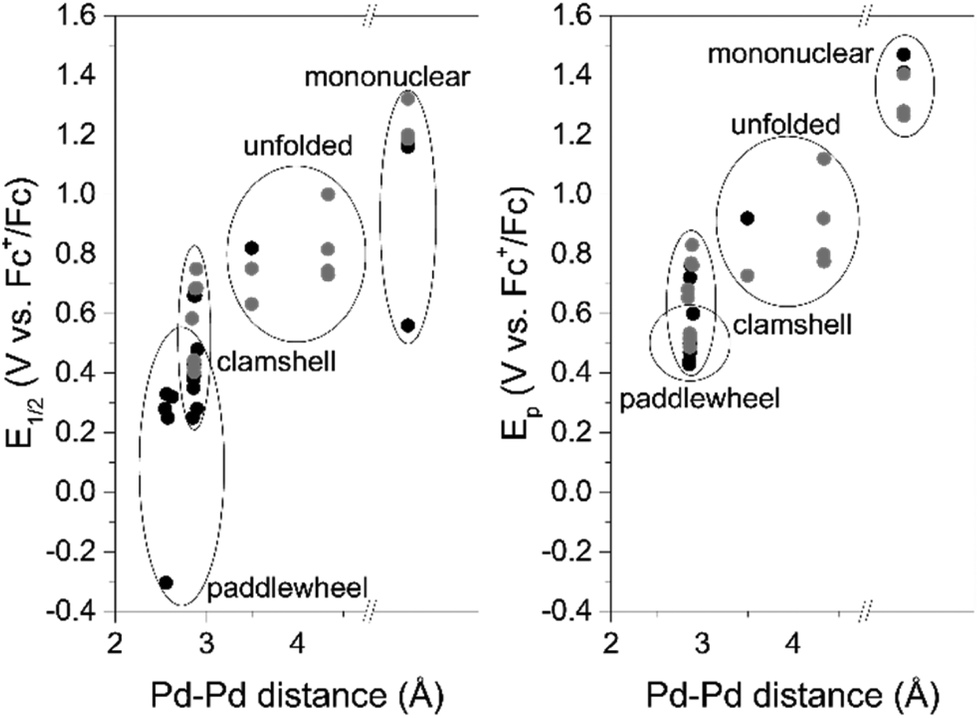
Redox trends in cyclometalated palladium( ii ) complexes - Dalton Transactions (RSC Publishing) DOI:10.1039/C6DT03786K
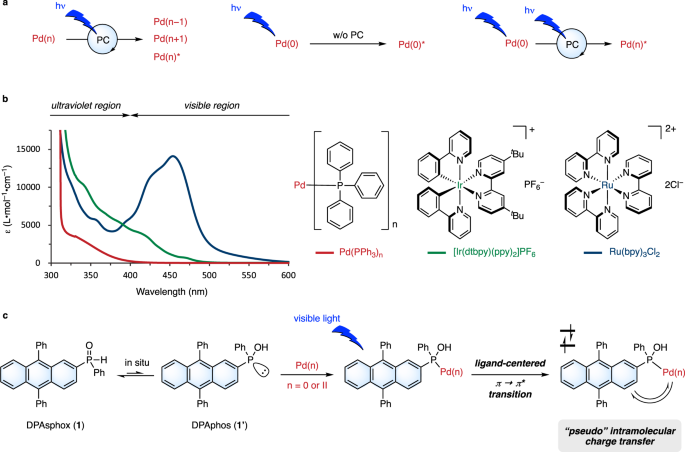
A visible-light activated secondary phosphine oxide ligand enabling Pd-catalyzed radical cross-couplings | Nature Communications

Accurate Oxidation Potentials of 40 Benzene and Biphenyl Derivatives with Heteroatom Substituents | The Journal of Organic Chemistry

Catalysts | Free Full-Text | Palladium-Based Catalysts as Electrodes for Direct Methanol Fuel Cells: A Last Ten Years Review
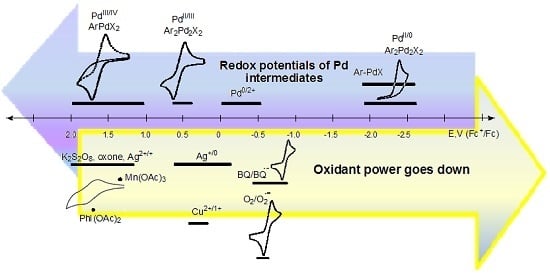
Inorganics | Free Full-Text | Redox-Induced Aromatic C–H Bond Functionalization in Metal Complex Catalysis from the Electrochemical Point of View
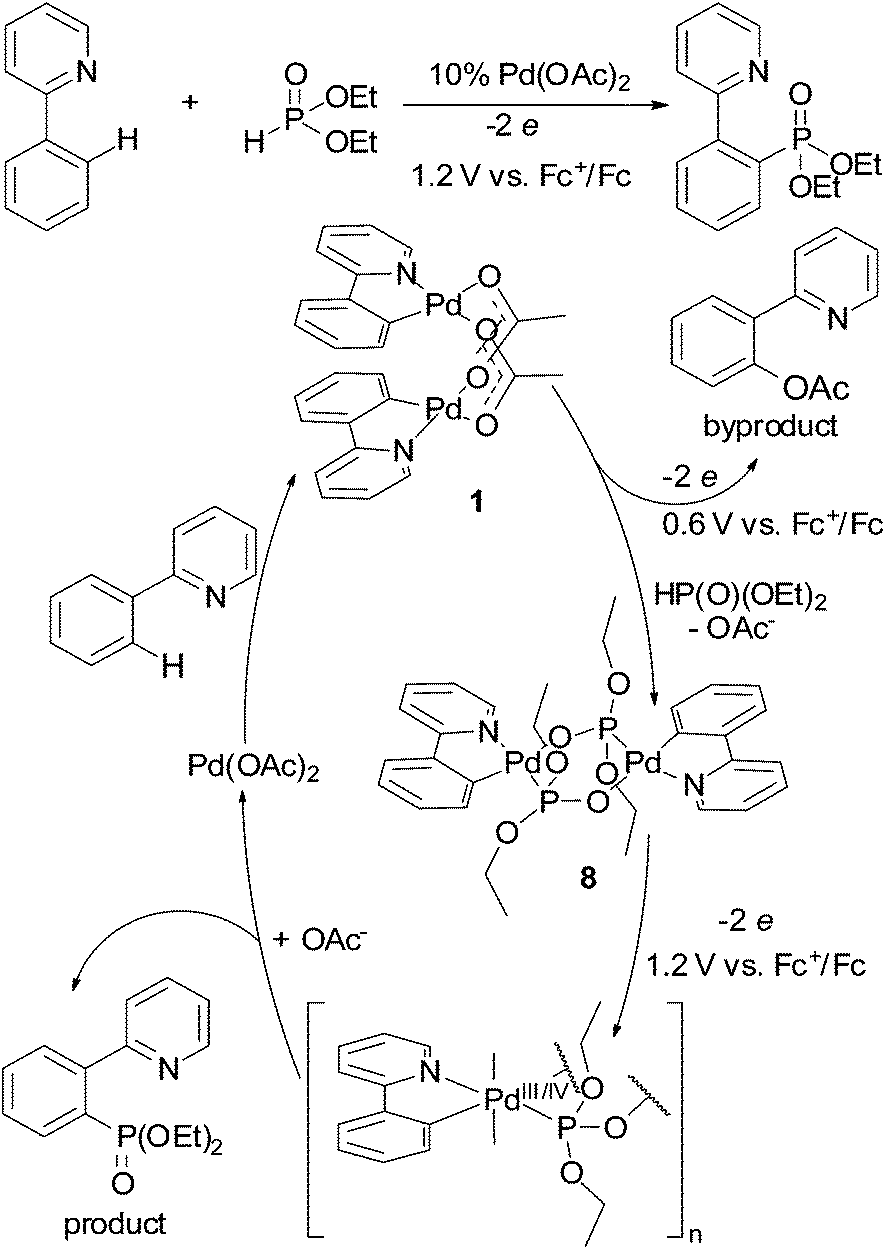
Redox trends in cyclometalated palladium( ii ) complexes - Dalton Transactions (RSC Publishing) DOI:10.1039/C6DT03786K